Conservation and Management of
Maldivian Mangrove Habitats – Baseline Study
Haa Alifu BAARAH
Table of Contents
2 Introduction
3 Overview of the island and social setting
3.1 Population and housing stats
3.2 Employment conditions
3.3 Cultural significance of Mangroves
4 Ecological Setting
4.1 Overview
4.2 Flora
4.2.1 Rhizophora mucronata (Ran’doo)
4.2.2 Ceriops tagal (Kanamana)
4.2.3 Lumnitzera racemosa (Burevi)
4.2.4 Rhizophora apiculata (Thakafathi)
4.2.5 Bruguiera cylindrica (Kan’doo)
4.2.6 Excoecaria agallocha (Thela)
4.2.7 Floral abundance and diversity
4.3 Fauna
4.3.1 Overview
5 Threats
5.1 Climate change
5.2 Anthropogenic
5.2.1 Existing
5.2.2 Potential
2 Introduction
Mangroves are woody plants that grow at the interface between land and sea in tropical and sub-tropical latitudes where they exist in conditions of high salinity, extreme tides, strong winds, high temperatures and muddy, anaerobic soils. There may be no other group of plants with such highly developed morphological and physiological adaptations to extreme conditions.
Mangrove forests occupy about 15 million hectares of tropical and subtropical coastline worldwide.
Although they amount to only 1 per cent of the total area of tropical forests, mangroves are highly productive ecosystems rich in biodiversity, consisting of a wide variety of plant species that provide important habitats for a wealth of fauna, including mammals, birds, reptiles, fish and molluscs. They contribute to livelihoods locally and globally by providing forest resources such as timber, firewood and thatching materials as well as non-timber products. Mangroves are also recognized as an important greenbelt and carbon sink that protects coastal areas from natural disasters such as tsunamis, cyclones and erosion resulting from sea-level rise, especially in small island countries.
Mangroves create unique ecological environments that host rich assemblages of species. The muddy or sandy sediments of the mangal are home to a variety of epibenthic, infaunal, and meiofaunal invertebrates. Channels within the mangal support communities of phytoplankton, zooplankton and fish. The mangal may play a special role as nursery habitat for juveniles offish whose adults occupy other habitats (e.g. coral reefs and seagrass beds).
As the mangroves are surrounded by loose sediments, the submerged mangroves’ roots, trunks and branches are islands of habitat that may attract rich epifaunal communities including bacteria, fungi, macroalgae and invertebrates. The aerial roots, trunks, leaves and branches host other groups of organisms. A number of crab species live among the roots, on the trunks or even forage in the canopy. Insects, reptiles, amphibians, birds and mammals thrive in the habitat and contribute to its unique character.
Living at the interface between land and sea, mangroves are well adapted to deal with natural stressors (e.g. temperature, salinity, anoxia, UV). However, because they live close to their tolerance limits, they may be particularly sensitive to disturbances like those created by human activities. Because of their proximity to population centers, mangals have historically been favored sites for sewage disposal. Industrial effluents have contributed to heavy metal contamination in the sediments. Oil from spills and from petroleum production has flowed into many mangals. These insults have had significant negative effects on the mangroves.
Maldives is known for its coral reefs and beaches; mangrove ecosystems in Maldives are over-shadowed by these environments and oftentimes neglected and under constant anthropogenic threats despite its crucial ecological and geomorphological function to this small island nation.
This study aims at establishing a baseline of environmental conditions of significant mangrove habitats throughout the country. Data and information collected via this study will provide a foundation for conservation efforts to build upon and help future environmental monitoring of said environments.
3 Overview of the island and social setting
3.1 Population and housing stats
HA. Baarah is located on the eastern rim of HA. Atoll at 6° 49.010’N and 73° 12.778’E. With a land area of 248.8 ha, this island is among the largest of HA. atoll. However, according to the latest available census data, the island of Baarah only account for 8.97% of the total atoll population even though it is the 4th most populated island of the atoll. With a recorded population of 1203 in 2006, the population density of the island was estimated to be 4.84 people per ha; 5th lowest of the atoll. Based on 2000 and 2006 census data, the annual population growth rate for Baarah was determined as -0.9. However, age-group population graphs showed a young population (majority of the population being 5-20 years old) and therefore population has increased exponentially since then. Population data recorded by the island council shows a registered population of 1948 by October 2014; a 62% increase in 8 years.
Figure 4‑1: Age group populations for Ha. Baarah (Census 2006)
As far as the island landuse patterns are considered, 16.4% of the total island is used for housing and residential purposes while roughly 6.2% of land has been cleared for agricultural purposes. Main residential area is located on the western side of the island extending from harbor towards south and north and slightly inland. At present there are 453 separate housing plots on the island.
Despite presence of numerous large wetland areas on Baarah, the community is unlikely to run out of land for housing in a near future. Nonetheless, a well developed landuse plan must be employed for proper use of land area on Baarah without threatening the wetland habitat.
3.2 Employment conditions
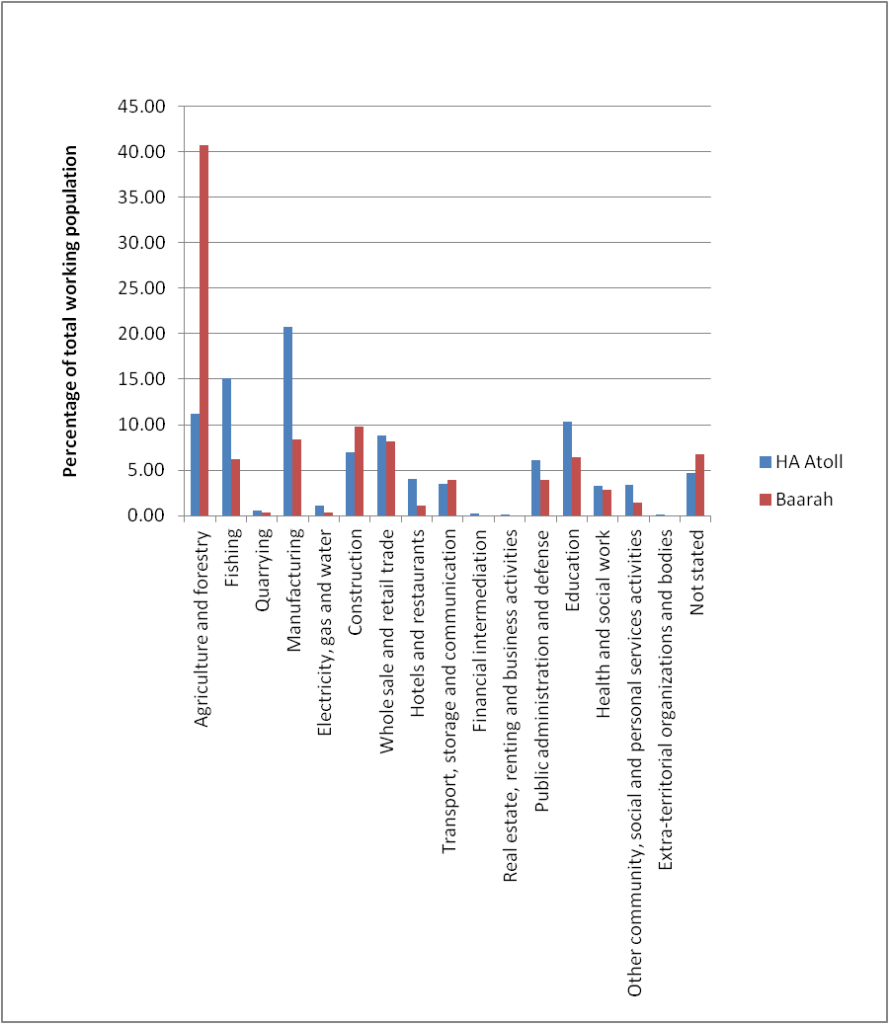
Based on the latest available census data, the most common occupations in Baarah community are in the agriculture and forestry industry. Majority of the people employed in this industry are skilled workers. In fact, the percentage of working population employed in this industry is significantly higher (almost 4 times) than the atoll average (Figure 4 -2).
Figure 4‑2: Employment of Baarah and HA Atoll communities by industry (percentage)
However, the link between this apparent increase in agricultural and forestry industry workers compared to the atoll average is not directly linked to the presence of large wetland areas on the island; rather, majority of these workers are thatch weavers. As such, at present, the wetland and mangrove habitats do not play a significant role in the island economy.
Nonetheless, from interviews with elderly members of the community, importance of wetland and related mangrove flora for the island economy in the past was evident. For instance, mangrove plants were cut down and sold as firewood and mangrove fruits were used as a food. The most common mangrove plant used/sold as firewood and as food source in the past was Kandoo (small leafed-orange mangrove- Bruguiera cylindrica). Burevi (Black mangroves- Lumnitzera racemosa) was also frequently used as firewood.
3.3 Cultural significance of Mangroves
One of the mangrove areas (Area B in Figure 5 -3) was famously used by as-Sulṭaan al-Ghaazee Muhamadhu Thakurufaanu al-A’uẓam as a hideaway for his boat, Kalhuoffummi during his campaign against Portuguese conquerors during 1570s.
Additionally, mangrove plants were used by the community in the past as a food source and firewood. Since the mangrove area B (Figure 5 -3) is directly connected to the lagoon, the waters are inhabited by medium sized reef fishes such as mullets and breams. According to the locals, people used to fish from this area frequently in the past, even though the practice is not that common nowadays.
4 Ecological Setting
4.1 Overview
There are 10 different water bodies (Figure 5 -3) on Baarah which can be considered different wetland areas, though some of which were originally single water bodies and later divided into separate areas from human and natural causes. Additionally, not all of these areas are mangrove habitats; Sites marked “A” and “I” did not have any flora or fauna associated with mangrove habitats meanwhile areas “C”, “D” and “E” had very few mangrove flora and fauna. The areas of these Sites are as below:
| Site |
Area (m2) |
|---|---|
| A | 22 100 |
| B | 133 990 |
| C | 63 710 |
| D | 57 150 |
| E | 23 610 |
| F | 72 580 |
| G | 5 370 |
| H | 8 290 |
| I | 3 080 |
| J | 2 790 |
The mangrove habitats were characterized by a central water body surrounded by mangrove plants, starting roughly 10m inwards from the waterline (at mean sea level) and extending roughly 20m outwards. Therefore, on average the mangrove flora belt is 30m wide across all the mangrove habitats on Baarah. However, it should be noted, on some occasions, (e.g. western section of Site B and north central section of Site F) mangrove flora belt was found to be as wide as 150m.
As far as the water quality of the sites are concerned; sites A, B, F and G were found to be saline (over 22ppt) while sites C, D and H were found to be brackish (Table 5 -1). Decaying plant matter and fine sediments were found suspended (in water column) in great concentrations at all the sites; leading to high TSS values. Typical values from on site measurements range from 55NTU to 100NTU for turbidity while TSS readings were found to range between 50mg/l to 80mg/l.
Table 5‑1: Water Quality readings
Sites B, C, D, E, F, G, H and J had an estimated water depth of 0.3 to 1m at MSL while Sites A and I had deep areas with depths of 1 to 2m at MSL. However, it should be noted; due to high amount of lose sediment and organic matter settled at the bottom, it is hard to quantify water depths accurately.
Sites B to H were found to have mud layers as thick as 0.7m around the water body. These mud layers are expected to contain an enormous amount of bacteria which breakdown organic matter and facilitate nutrient recycling. In fact, mangrove bacterial communities are found to be among the most productive bacterial communities.
Site B, in addition to housing an abundance of mangal vegetation, is directly connected to the adjacent lagoon and marine habitat; making it one of the most important habitats on the island in terms of ecological value. Mangrove plants and their roots provide structure and channels for juvenile marine organisms such as fish and crustaceans to seek refuge in. Furthermore, mangrove plants and related fauna provide juvenile marine organisms with food; forming a direct link between terrestrial nutrients and marine environment.
Sites C to F are inhabited by a large population of tilapia (Oreochromis sp.). Even though they not a native species of fish, they are an invaluable source of food for the avian community as can be seen by the large number of birds foraging at these sites.
Figure 5‑1: Wetland Areas and general landuse of HA. Baarah
4.2 Flora
Six species of mangrove plants were found in Baarah during the field survey; Rhizophora mucronata (Red mangrove- Randoo), Ceriops tagal (Yellow Mangrove- Karamana) Lumnitzera racemosa (Black mangrove- Burevi), Rhizophora apiculata (Tall stilted mangrove- Thakafathi), Bruguiera cylindrica (small- leafed orange mangrove- Kandoo) and Excoecaria agallocha (Milky mangrove- Thela).
4.2.1 Rhizophora mucronata (Ran’doo)
| Scientific Name: | Rhizophora mucronata |
| Common Name: | loop-root mangrove, red mangrove or Asiatic mangrove |
| Conservation status: | Currently categorized as of Least Concern according to the IUCN Red List of Threatened Species. |
| Habitat: | This species is found in the intermediate to upstream estuarine zone in the lower to mid-intertidal region, and more to the seaward side. This species tolerates a maximum salinity of 40 ppt and a salinity of optimal growth of 8-33 ppt. |
| Key Uses: | Timber, charcoal, medicine, coastal erosion control, nursery grounds for fish. |
| Sites: | B C F G H |
| Taxonomy | |
| Class: | Equisetopsida |
| Subclass: | Magnoliidae |
| Superorder: | Rosanae |
| Order: | Malpighiales |
| Family: | Rhizophoraceae |
| Genus: | Rhizophora |
Morphology:
A much branched large shrub or moderate sized tree, up to 10 m tall, supported on adventitious prop roots from stem and branches with reddish brown bark. Leaves with 1-3 cm long, stout petiole; lamina broadly elliptic or oblong-elliptic to oval, (6-) 8-15 (-20) cm long. Branches horizontal; trunk and lower branches supported by numerous profusely looping stilt-roots and prop roots, lenticellate, bark brown, longitudinally fissured; branchlets, terete, brownish-green, glabrous, with prominent, rough, thick, brown layer of stipular scar, prominent.
4.2.2 Ceriops tagal (Karamana)
| Scientific Name: | Ceriops tagal |
| Common Name: | Indian mangrove, Yellow mangrove |
| Conservation status: | Currently categorized as of Least Concern according to the IUCN Red List of Threatened Species. |
| Habitat: | This species is found from downstream to intermediate estuarine zones in the mid to high intertidal regions. It is shade intolerant with a maximum porewater salinity of 45 ppt and a salinity of optimal growth of 0-15 ppt |
| Key Uses: | The trunk is used in house building and for fuel, bark for tanning and the fruits are sometimes eaten |
| Sites: | B |
| Taxonomy | |
| Class: | Eudicots |
| Order: | Malpighiales |
| Family: | Rhizophoraceae |
| Genus: | Ceriops |
Morphology:
Evergreen tree 5–15(-25) m high often with unbranched stilt roots and thin knees 20–30 cm high. Bark; light gray or reddish-brown, smooth or irregularly fissured; inner bark orange or reddish. Leaves opposite, clustered at end of twigs, obovate to elliptical, 5–10 cm long, 2–6 cm wide, rounded and emarginate at tip, acute at base, entire, thick, leathery, glabrous, without visible veins. Petiole 1–3.5 cm long, stipules paired, narrow, ca 2 cm long. Cymes single and short-stalked in leaf axils. Flowers 4–10, short stalked, ca 6 mm long. Calyx yellow-green with 5–6 narrow pointed lobes turned back on fruit; petals 5–6, white, united at base, 2-lobed and ending in 2–4 bristles, stamens 10–12; pistil with conical, partly inferior 3-celled ovary and short style. Berry drooping, ovoid, 1.5–2.5 cm long, leathery. Seed 1, viviparous, becoming cigar-shaped or club-shaped, sharply angled, 15–25(-35) cm long.
4.2.3 Lumnitzera racemosa (Burevi)
| Scientific Name: | Lumnitzera racemosa |
| Common Name: | Black Mangrove |
| Conservation status: | Currently categorized as of Least Concern according to the IUCN Red List of Threatened Species. |
| Habitat: | Usually restricted to the landward edge of open mangrove forests, Lumnitzera racemosa is found along coastal shores, lagoons, saltwater and freshwater swamps, swampy meadows and in sandy soils |
| Key Uses: | The timber is used in salt water piling and fencing. It is also used for house posts, boat building and as firewood. |
| Sites: | B |
| Taxonomy | |
| Class: | Eudicots |
| Order: | Myrtales |
| Family: | Combretaceae |
| Genus: | Lumnitzera |
Morphology:
Its tree bark is brown colored and ‘knee roots’ are often formed through the extension of the curved aerial roots. The leaves, which are alternately arranged and emerald-colored, grow in clusters at the tip of the branch. The leaf margin is wavy with small serrations. The obovate leaf is either round or caved at the front, similar to a mullet roe. The flower has five white-colored petals and there are two blooming periods each year. The first flowering period occurs between May and July, while the second time occurs between October and November. The fruits of Lumnitzera racemosa are drupes, whose endocarp is solid and expcarp is similar to that of a sponge. This allows the fruit to float on the water surface and be distributed through water dispersal. Lumnitzera racemosa is the most salt-tolerant tree species amongst the mangrove plants. Its roots develop into ‘knee roots’, which provides stability.
4.2.4 Rhizophora apiculata (Thakafathi)
| Scientific Name: | Rhizophora apiculata |
| Common Name: | Tall-stilt Mangrove |
| Conservation status: | Currently categorized as of Least Concern according to the IUCN Red List of Threatened Species. |
| Habitat: | This species is found in the intermediate estuarine zone in the mid-intertidal region. This species tolerates a maximum salinity of 65 ppt and a salinity of optimal growth of 8-15 ppt. Sediment accretion increases the mortality rate of seedlings. This species will not be an efficient colonizer of coastal areas exposed to sudden discharges of sediments such as those of highly eroding watersheds |
| Key Uses: | The timber is used in salt water piling and fencing. It is also used for house posts, boat building and as firewood. |
| Sites: | B |
| Taxonomy | |
| Class: | Eudicots |
| Order: | Malpighiales |
| Family: | Rhizophoraceae |
| Genus: | Rhizophora |
Morphology:
Trees are 20-30m tall. Bark dark grey and chequered. Conspicuous arching stilt roots that can extend 5m up the stem. Often also with lots of aerial roots emerging from the branches so that the tree appears to have a skirt of roots under the leaves.
Leaves eye-shaped (8-15cm long), glossy green and stiff, with tiny evenly distributed black spots on the underside. Stipule is usually (but not always) red.
Flowers (1-2cm) in pairs on very short stalks so they appear to be stuck directly onto the branch. Calyx globular hard thick, brown on the outside yellow inside. Petals yellow to white, flat membranous and hairless, falling off soon after blossoming.
The fruit looks like a brown, upside down pear (about 2cm) and is crowned by short persistent sepals. The cylindrical hypocotyl can be up to 38cm long, somewhat smooth, green ripening purple.
4.2.5 Bruguiera cylindrica (Kan’doo)
| Scientific Name: | Bruguiera cylindrica |
| Common Name: | Small-leafed Orange Mangrove, Reflexed Orange Mangrove |
| Conservation status: | Currently categorized as of Least Concern according to the IUCN Red List of Threatened Species. |
| Habitat: | This species is found in downstream and intermediate estuarine zones in the mid-intertidal region. This species is also shade tolerant. |
| Key Uses: | Fruit is used for human consumption while timber is sometimes used as firewood. |
| Sites: | D E H G |
| Taxonomy | |
| Class: | Eudicots |
| Order: | Malpighiales |
| Family: | Rhizophoraceae |
| Genus: | Bruguiera |
Morphology:
Bruguiera cylindrica is a columnar tree growing to 23 m high. It has finely fissured, greyish bark. There are short buttresses at the base of the trunk and small, knee-like air-breathing roots (pneumatophores).
The leaves are simple, opposite, thin and glossy green, elliptic in shape, 5 – 17 cm long, 2 – 8 cm wide, with a bluntly pointed apex. The leaves occur in clusters at the end of branches. The petiole is often reddish and 1 – 4.5 cm long.
The inflorescence is often three-flowered and axillary. Flowers have a pale-greenish calyx with 8 calyx lobes, 16 stamens and 8 white, bi-lobed petals with 2 – 3 bristles on each apex and 1 conspicuous bristle in the sinus (indentation between the petal lobes) that is longer than the petal lobes. The viviparous propagule grows from within the calyx and is pencil-like and green, with a smooth surface. It is 8 – 15 cm long and 0.4 – 0.8 cm wide.
4.2.6 Excoecaria agallocha (Thela)
| Scientific Name: | Excoecaria agallocha |
| Common Name: | Milky Mangrove, Blind-your eye Mangrove, river poison tree |
| Conservation status: | Currently categorized as of Least Concern according to the IUCN Red List of Threatened Species. |
| Habitat: | This is a back mangrove species and often exploits open areas and is tolerant of distrurbed areas. |
| Key Uses: | This species is used for furniture and ornaments. In Papua New Guinea it is used for pain relief from fish stings. It is also used as a poison to catch fish. Used in traditional medicine and also as firewood. |
| Sites: | F, G |
| Taxonomy | |
| Class: | Eudicots |
| Order: | Malpighiales |
| Family: | Euphorbiaceae |
| Genus: | Excoecaria |
Morphology:
The tree grows to 15m tall sometimes branched at the base, thus forming multiple trunks. Roots run along the ground surface and often knotted and covered with lenticels. The tree does not have root systems obviously specialized for mangroves and that it is also found to an elevation of 400m and thus cannot be regarded as an exclusively mangrove tree. The tree bark is grey, smooth but warty, becoming fissured. Lenticels are prominent on young twigs.
Leaves are thick, oval and pointed (5-10cm long), arranged alternately in a spiral. Young leaves are pink, old leaves turn yellow then red before dropping off. Leaves usually drop off after dry weather.
Flowers are tiny (less than 1mm). Trees bear either male or female flowers, never both. Male flowers start as upright narrow cones when young and as they develop, elongate into longer spikes (5-10cm) that eventually form drooping yellow tassels. Female flowers appear in shorter spikes.
The fruits are small (less than 1cm) three-lobed, green turning black as they ripen into dry capsules. Each capsule is made up of three portions, containing tiny dark to black seeds.
4.2.7 Floral abundance and diversity
Site A:
No mangrove flora was observed at this site. The flora around water body consist of typical coastal vegetation found in Maldivian islands; mostly iron wood trees on the seaward side while sea hibiscus, sea lettuce and wild screw-pines were dominant on the landward side.
Site B:
An abundance of mangrove flora was observed on this site with different areas dominated by different species of mangroves (). However, the most common mangrove species found at this site was Lumnitzera racemosa, Black Mangroves. They dominated the south and south west side of the water body while Rizophora spp. dominated the north and north east side. A patch of Ceriops tagal, the Indian Mangrove was found on the northern side of water body.
Shannon’s diversity index (H) and evenness (EH) was calculated for Site B using data collected from vegetation transects;
H = 1.27
EH = 0.71
Figure 5‑4: Results of Vegetation Transects; Site B
Sites C, D and E:
Despite the presence of the large wetland area; mangrove flora at these sites was very limited. Only two species of mangrove plants were found at these 3 sites; Rizophora mucronata and Buruguiera cylindrica. Few trees (less than 15) of R. mucronata were seen around the centre of Site C while up to 20 or so individual trees of B. cylindrica were seen between E and D. Furthermore, small buds of R. mucronata were seen to have been planted by the community towards the northern end of Site C.
Site F:
Only two species of mangrove flora was found at this site; Excoecaria agallocha, Milky mangroves and R. mucronata, red mangroves. R. mucronata was only found at the northern tip of the water body whereas E. agallocha was seen around the water body frequently. A notable increase in the amount of E. agallocha was seen on the eastern side of the water body compared to western side; roughly 15m tall trees every 10m or so were seen on the western side while 5m tall trees every 3 to 5m were seen on the eastern side. At the northern tip of the water-body R. mucronata were seen to make up to 95% of the vegetation (roughly 3 trees per sq.m).
Site G:
Three species of mangrove flora was found at this site; R. mucronata, E. agallocha and B. cylindrica; however, R. mucronata were the most common making up to 80% of the mangrove plants at Site G. The trees were sparsely distributed around the water body; 3 to 8m tall, roughly 1 tree per 10 sq.m.
Site H:
Two species of mangrove flora, E. agallocha and B. cylindrica were found at this site, E. agallocha being the most dominant. Only few mature trees of B. cylindrica were seen at this site while E. agallocha were common; making up 30% of total vegetation (up to 20m from water line). Patches of E. agallocha had about 1 tree per sq.m. Rest of the vegetation mainly consist of sea hibiscus (Hibiscus tiliaceus) and coconut palms (Cocus nucifera).
Site I:
There was no mangrove flora at this site. The vegetation around the water-body was predominantly made up of sea hibiscus (Hibiscus tiliaceus) and sea lettuce (Scaevola taccada).
Site J:
Only one species of mangrove flora was found at this site; B. cylindrica. Seven trees of B. cylindrica; about 5m tall, were seen on the western side of the water body. Pandunus tectoris and Scaevola taccada were the most common trees at this site, making up 90% of the total vegetation.
4.3 Fauna
4.3.1 Overview
An abundance of mangrove fauna was observed in Baarah wetland areas; these include crustaceans, aves and insects. The most common fauna observed were mangrove crabs and hermit crabs. Additionally, tree ants, grey herons were seen foraging in the wetland areas in abundance.
Crustacea
In many mangroves a large proportion of the leaf litter is directly consumed by crabs, particularly those in the family Sesarmidae. This dramatically accelerates the incorporation of mangrove biomass into the food chain. The acceleration happens in three main ways:
1. Shredding – as crabs feed on leaf litter they shred it into fine particles, increasing the surface area for leaching and microbial colonisation. An Australian study found that 20% of the material processed by crabs is dropped without being ingested (Camilleri 1989), but even this is shredded into fine particles.
2. Accelerated leaching – 85% of the leaf litter ingested by crabs ends up in faeces. Processing of this material in the crab gut reduces content of unpalatable tannins in faeces to less than 3%, compared to 13% in freshly fallen leaves (Lee 1998). This process means that decomposing microbes can colonise the processed leaves in hours or days, rather than the weeks required without crabs.
3. Assimilation – around 12% of the leaf litter processed by crabs is assimilated as crab biomass. A range of predators then feed on these crabs, including a number of fish species that are of high importance to fisheries (Sheaves and Molony 2000).
Additionally, crabs will invest a proportion of the energy assimilated in reproduction, producing large numbers of crab larvae which are an important food source to smaller predators.
Estimates for the amount of litter consumed by crabs vary. In many mangroves, crabs play a major role: one Australian study found that 70% of Bruguiera leaves and 88% of Ceriops leaves were taken down crab burrows in an Australian mangrove, and those left on the surface were eaten by crabs where they fell (Robertson and Daniel 1989b). Similar results have been found in Brazil, where the crab Ucides cordatus was found to consume 84.2% of the total daily litterfall (Nordhaus et al. 2006).
Mangrove crabs and hermit crabs were in abundance at all wetland areas of Baarah; particularly at site B.
Aves
A significant number of Common Greenshank (Tringa nebularia) was observed at Sites C, D and E; few individuals were also observed at other sites.
Other fauna
As the water body in Site A and B were directly linked to the lagoon, a significant amount of marine fauna such as Sting Rays, some species of reef fish (particularly juveniles in abundance) were seen during the time of survey. In addition, groups of ghost shrimps were also seen at Site B. A congo eel (Amphiuma sp.) was sighted at Site F.
5 Threats
5.1 Climate change
Among the predicted outcomes of climate change, the most pronounced effect is sea-level rise due to global warming. Mangrove systems do not keep pace with changing sea-level when the rate of change in elevation of the mangrove sediment surface is exceeded by the rate of change in relative sea-level. The understanding of mangroves as opportunistic colonizers with distribution controlled through ecological responses to environmental factors highlights the importance of the geomorphic setting in determining where mangrove ecosystems establish, their structure and functional processes. An understanding of a mangrove’s geomorphic setting, including sedimentation processes (sediment supply and type), hydrology, and energy regime, is likewise important in understanding resistance and responses to changes in sea-level, as these affect both surface and subsurface controls on elevation of the mangrove sediment surface.
5.2 Anthropogenic
5.2.1 Existing
Due to extensive fresh water bodies in Baarah, mosquitoes used to breed in them in astounding numbers. This lead to community of the island to dig channels from the water bodies in to the lagoon, allowing sea water to enter. This changed salinity of water body significantly; previous freshwater water bodies (sites marked C, D and E) now house brine; possibly effecting flora and fauna composition. Additionally, invasive and territorial fish species, Tilapia has been introduced into these water bodies as well. Their numbers exploded and has most likely eradicated any potential native fish species in the water bodies.
Even though in the past, the local community used to collect firewood and timber from mangroves around Site B, this activity is reduced greatly at present.
An experimental aquaculture activity was initiated in Site C, potentially altering the natural bathymetry of the water body. Site I has been dug in the past according to the locals.
Overall, Sites A, B, F, G, H and J is not altered by anthropogenic activities to a significant level, however, Sites C, D, E and I have been greatly altered in the past.
5.2.2 Potential
Agricultural and industrial work is ever-expanding on Baarah; some of these are in very close proximity to the wetland areas. If not managed, these could pose a significant threat to mangrove habitats via pollution and deforestation.